Search Result
Results for "
nucleophiles
" in MedChemExpress (MCE) Product Catalog:
3
Biochemical Assay Reagents
3
Isotope-Labeled Compounds
| Cat. No. |
Product Name |
Target |
Research Areas |
Chemical Structure |
-
- HY-W009177
-
|
|
Glyoxalase (GLO)
|
Others
|
|
S-Methylglutathione is an S-substitued glutathione and a stronger nucleophile than GSH . S-Methylglutathione has inhibitory effect on glyoxalase 1 .
|
-

-
- HY-N0548
-
|
|
Glutathione S-transferase
|
Cancer
|
|
α-Angelica lactone is a naturally occurring anticarcinogen and an vinylogous nucleophile. α-Angelica lactone can give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives and exhibitselectrophilic trapping at the γ-carbon. α-Angelica lactone exerts strong chemoprotective effects by selective enhancement of glutathione-S-thansferase (GST) and UDP-glucononosyltransferase (UGT) detoxification enzymes .
|
-

-
- HY-20556
-
-

-
- HY-18081
-
|
|
FAAH
Autophagy
|
Metabolic Disease
|
|
PF 750 is a selective and covalent fatty acid amide hydrolase (FAAH) inhibitor, with IC50s varied from 16.2-595 nM in different pre-incubation times. Covalently modifies the enzyme’s active site serine nucleophile .
|
-

-
- HY-122426
-
|
n-Undecyl bromide
|
Biochemical Assay Reagents
|
Others
|
|
1-Bromoundecane (n-Undecyl bromide) is a chemical reagent featuring a bromide along a saturated C11 chain. Bromide is easily displaced by nucleophiles such as alcohols or amines. Compounds such as this may be used as intermediates in building lipids for use in lipid nanoparticles.
|
-

-
- HY-131846
-
|
6-Chloropurine 5'-ribonucleotide
|
Nucleoside Antimetabolite/Analog
|
Others
|
|
6-Cl-5'-PuMP is a reactive analogue of adenosine 5’-O-monophosphate (5’-AMP), and is suitable for modifying position 6 using various nucleophiles .
|
-

-
- HY-N0548R
-
|
|
Glutathione S-transferase
|
Cancer
|
|
α-Angelica lactone (Standard) is the analytical standard of α-Angelica lactone. This product is intended for research and analytical applications. α-Angelica lactone is a naturally occurring anticarcinogen and an vinylogous nucleophile. α-Angelica lactone can give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives and exhibitselectrophilic trapping at the γ-carbon. α-Angelica lactone exerts strong chemoprotective effects by selective enhancement of glutathione-S-thansferase (GST) and UDP-glucononosyltransferase (UGT) detoxification enzymes .
|
-

-
- HY-W800830
-
|
|
Biochemical Assay Reagents
|
Others
|
|
DMG-Nitrophenyl Carbonate is a short reagent featuring a DMG lipid headgroup and a nitrophenyl carbonate, which is readily displaced by amine nucleophiles to form carbamate bonds under mild conditions.
|
-

-
- HY-W591314
-
|
|
Biochemical Assay Reagents
|
Others
|
|
m-PEG11-Ms is a PEG linker featuring a methoxy cap and a mesylate group. The methoxy cap is inert while the mesylate is a good leaving group which is easily displaced by nucleophiles such as alcohols and amines.
|
-

-
- HY-W586329
-
|
|
Ligands for E3 Ligase
|
Others
|
|
Thalidomide-4-carbaldehyde is a Thalidomide analogue with an aldehyde. Thalidomide recruiits E3 ligase for the ubiquitinylation and subsequent proteolysis of target proteins. The aldehyde is highly reactive towards amine nucleophiles through reductive amination among other reactions.
|
-

-
- HY-W451211
-
|
|
Ligands for E3 Ligase
|
Cancer
|
|
Thalidomide-5-carbaldehyde is a Thalidomide analogue with an aldehyde. Thalidomide recruiits E3 ligase for the ubiquitinylation and subsequent proteolysis of target proteins. The aldehyde is highly reactive towards amine nucleophiles through reductive amination among other reactions.
|
-

-
- HY-W393115
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Diethyl 6-bromohexylphosphonate is a short linker featuring a bromide and a diethyl phosphonate. The bromide is a good leaving group which can be easily displaced by nucleophiles like alcohols, amines or thiols, while the diethyl phosphonate may be hydrolyzed to allow for esterification.
|
-

-
- HY-W190836
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Azide-PEG2-Tos is a small PEG linker featuring an azide and a tosylate. Tosylates are good leaving groups which are easily displaced by nucleophiles while azide is a click chemistry handle which is used to react with terminal alkynes or strained cyclooctynes.
|
-

-
- HY-W190738
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Azide-PEG8-Tos is a small PEG linker featuring an azide and a tosylate. Tosylates are good leaving groups which are easily displaced by nucleophiles while azide is a click chemistry motif which is used to react with terminal alkynes or strained cyclooctynes.
|
-

-
- HY-172511
-
|
|
Biochemical Assay Reagents
|
Others
|
|
m-PEG9-4-nitrophenyl carbonate serves as a PEG linker containing a nitrophenyl carbonate, which is readily displaced by amine nucleophiles to form carbamate bonds under mild conditions. The PEG9 chain bolsters the water solublity of the compound.
|
-

-
- HY-W800743
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Heptyl 6-bromohexanoate is a chemical reagent featuring a bromide linked to a larger ester chain consisting of a C6 ester coupled to a C7 alcohol. Bromide is easily displaced by nucleophiles in substitution reactions. Compounds such as this may be used as intermediates in building lipids for use in lipid nanoparticles.
|
-

-
- HY-150226
-
|
|
Proteasome
|
Inflammation/Immunology
|
|
Enzyme-IN-1 (compound 1) is a peptide-based inhibitor of N-terminal nucleophile (Ntn) hydrolases. Specifically, Enzyme-IN-1 inhibits the chymotrypsin-like activity (CT-L) of the 20S proteasome. Enzyme-IN-1 may has potential antiinflammatory properties .
|
-

-
- HY-W190936
-
|
|
E3 Ligase Ligand-Linker Conjugates
|
Cancer
|
|
Thalidomide-O-PEG5-Tosyl is a molecule that incorporatess the Thalidomide based cereblon ligand and 4-unit PEG linker used in PROTAC technology. Thalidomide-O-PEG5-tosyl is reactive with nucleophiles such as amine, hydroxy containing molecules.
|
-

-
- HY-W440809
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Nonyl 8-bromooctanoate is a chemical reagent featuring a bromide linked to a larger ester chain consisting of a C8 ester coupled to a C9 chain. Bromide is easily displaced by nucleophiles in substitution reactions.Nonyl 8-bromooctanoate can be used as an intermediate in building lipids for use in lipid nanoparticles.
|
-

-
- HY-W800652
-
|
|
PROTAC Linkers
|
Cancer
|
|
(S, R, S)-AHPC-PEG4-tosyl is a PROTAC linker that incorporatess an E3 ligase ligand with a PEG4 linker to empower PROTAC drug research & discovery. PEG4 spacer increases the compound's hydrophility. Tosyl group is reactive with amine or other nucleophiles.
|
-

-
- HY-W401062
-
|
1,2-Epoxytridecane
|
Biochemical Assay Reagents
|
Others
|
|
2-Undecyloxirane (1,2-Epoxytridecane) is a chemical reagent featuring an epoxy group on a C11 chain. The epoxy group undergoes ring opening in the presence of nucleophiles to form a branched structure containing a secondary alcohol. Compounds such as this may be used as intermediates in building lipids for use in lipid nanoparticles.
|
-

-
- HY-128429R
-
|
|
Fungal
Biochemical Assay Reagents
|
Infection
|
|
Trans-2-Hexenal (Standard) is the analytical standard of Trans-2-Hexenal. This product is intended for research and analytical applications. Trans-2-Hexenal can be used for the determination of low-molecular-weight carbonyl compounds which are reactive with biological nucleophiles in biological samples .
|
-

-
- HY-W800742
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Heptadecan-9-yl 6-bromohexanoate is a chemical reagent featuring a bromide linked to a larger ester chain consisting of a C6 ester coupled to the central position of a C17 chain. Bromide is easily displaced by nucleophiles in substitution reactions. Compounds such as this may be used as intermediates in building lipids for use in lipid nanoparticles.
|
-

-
- HY-126944
-
|
2-Amino-N-phenylbenzamide
|
Biochemical Assay Reagents
|
Cancer
|
|
2-Aminobenzamide is a neutral and stable compound used as fluorescent tag, numerously in Glycan analysis. 2-aminobenzamide acts as the starting material for several important reactions like Bargellini reaction as an competent ambident nucleophile. Specifically 2-aminobenzamide and its derivatives are used in the blood coagulation cascade .
|
-

-
- HY-14380
-
PF-3845
1 Publications Verification
|
FAAH
Autophagy
|
Inflammation/Immunology
Cancer
|
|
PF-3845 is a potent, selective, irreversible and orally active inhibitor of fatty acid amide hydrolase (FAAH), with a Ki of 0.23 μM. PF-3845 is a covalent inhibitor that carbamylates FAAH's serine nucleophile. PF-3845 can reduce pain sensation, inflammation, and anxiety/depression without substantial effects on motility or cognition .
|
-

-
- HY-101974
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Biotin-PEG3-Bromide is a short PEG linker featuring a biotin group and a bromide. The bromide is a halogen which is easily displaced by nucleophiles such as alcohols or amines. Alternatively, bromide can be applied in a number of cross-coupling reactions such as in a Suzuki reaction. Biotin is useful for affinity-based applications such as pull-down assays or for ligating with streptavidin proteins.
|
-

-
- HY-172294
-
|
|
Biochemical Assay Reagents
|
Others
|
|
Mes-PEG2-CH2-t-butyl ester is a PEG linker consisting of a PEG2 linker for improved water-solubility of the compound and a t-butyl ester group which can be deprotected under acidic conditions. The mesylate serves as an excellent leaving group, offering all the advantages without the limitation of having an acidic proton that could react with nucleophiles.
|
-

-
- HY-112526
-
|
|
Fluorescent Dye
|
Others
|
|
Thiofluor 623 (Compound 3) is a fluorescent turn-on probe that can be used for the selective sensing and bioimaging of thiols. Thiofluor 623 displays excellent immunity to interference from nitrogen and oxygen nucleophiles. Thiofluor 623 is essentially nonfluorescent in the absence of thiols, which cleave the probe and release the red-emissive donor-acceptor fluorophore (Ex=563 nm, Em=623 nm) .
|
-

-
- HY-W728451
-
|
|
FAAH
|
Cardiovascular Disease
Neurological Disease
|
|
URB694 is a carbamate FAAH inhibitor that irreversibly carbamoylate the nucleophile catalytic serine in FAAH active site. URB694 exhibits antidepressant-like activity and cardioprotective effects. URB694 can be used to prepare 11C-Carbonyl-URB694 for in vivo positron emission tomography (PET) imaging studies of the brain FAAH .
|
-

-
- HY-W800844
-
|
|
Biochemical Assay Reagents
|
Others
|
|
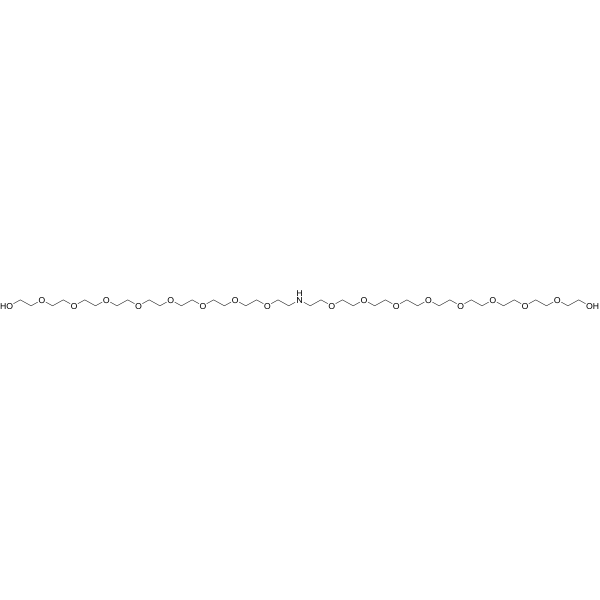
NH-bis(PEG8-OH) is a homobifunctional reagent containing two alcohols joined together by a secondary amine. The alcohols can be used in a variety of ways such as in forming esters with carboxylic acids, while the secondary amine can be used as a nucleophile in linking with ketones, aldehydes, and carboxylic acids. The PEG spacers make this molecule water-soluble, potentially altering its DMPK properties.
|
-
-
- HY-28009
-
|
|
Biochemical Assay Reagents
|
Others
|
|
2-(7Z,10Z)-7,10-Hexadecadien-1-yloxirane is an intermediate for the preparation of lipid molecules. 2-((7Z,10Z)-Hexadeca-7,10-dien-1-yl)oxirane features a polyunsaturated chain with an epoxy ring that can be opened by nucleophiles.
|
-

-
- HY-137383
-
|
|
Biochemical Assay Reagents
Reactive Oxygen Species (ROS)
|
Others
|
|
Sulfo-SANPAH is a primary amine-nitrobenzene azide cross-linker. Sulfo-SANPAH is widely used to crosslink ECM proteins to various substrates, including acrylic-based hydrogels, such as polyacrylamide hydrogels. Sulfo-SANPAH facilitates covalent binding through its negatively charged sulfonate group on its N-hydroxysuccinimide ester ring and a photoactivated phenyl azide group that is highly reactive with nucleophiles and free radicals .
|
-

-
- HY-106991A
-
|
S-303 dihydrochloride
|
HIV
Bacterial
CHIKV
|
Infection
|
|
Amustaline (S-303) dihydrochloride, a nucleic acid-targeted alkylator, is an efficient pathogen inactivation agent for blood components containing red blood cells. Amustaline dihydrochloride has three components: an acridine anchor (an intercalator that targets nucleic acids non-covalently), an effector (a bis-alkylator group that reacts with nucleophiles), and a linker (a small flexible carbon chain containing a labile ester bond that hydrolyzes at neutral pH to yield non-reactive breakdown products) .
|
-

-
- HY-W073382
-
|
Bis–sulfone Acid
|
Biochemical Assay Reagents
|
Others
|
|
4-(3-Tosyl-2-(tosylmethyl)propanoyl)benzoic acid (Bis-sulfone Acid) is a strong covalent linker featuring a free carboxylic acid and two tosyl groups. Each tosyl group can be displaced by thiol or amine nucleophiles via a Michael addition, and the inclusion of two on this molecule allow this reaction to proceed twice. This may be used to “staple” two reduced cysteine residues on a given protein to reform disulfide bridges. The carboxylic acid is free to react to form amides or esters.
|
-

-
- HY-128429
-
|
(E)-2-Hexenal
|
Fungal
Biochemical Assay Reagents
|
Infection
|
|
trans-2-Hexenal ((E)-2-Hexenal) is a volatile compound widely present in fresh plants, vegetables, and fruits, with a unique leafy aroma. trans-2-Hexenal has antifungal activity and can also inhibit the germination of soybean seeds and the growth of seedlings. In addition, trans-2-Hexenal can be used to determine low-molecular-weight carbonyl compounds that are reactive with biological nucleophiles in biological samples .
|
-

-
- HY-106991AR
-
|
|
HIV
Bacterial
Reference Standards
CHIKV
|
Infection
|
|
Amustaline (dihydrochloride) (Standard) is the analytical standard of Amustaline (dihydrochloride). This product is intended for research and analytical applications. Amustaline (S-303) dihydrochloride, a nucleic acid-targeted alkylator, is an efficient pathogen inactivation agent for blood components containing red blood cells. Amustaline dihydrochloride has three components: an acridine anchor (an intercalator that targets nucleic acids non-covalently), an effector (a bis-alkylator group that reacts with nucleophiles), and a linker (a small flexible carbon chain containing a labile ester bond that hydrolyzes at neutral pH to yield non-reactive breakdown products) .
|
-

-
- HY-10865
-
|
|
FAAH
Autophagy
|
Neurological Disease
|
|
LY2183240 is a highly potent blocker of anandamide uptake (IC50= 270 pM; Ki=540 nM). LY2183240 is a potent, covalent inhibitor of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) with an IC50 of 12.4 nM. LY2183240 inactivates FAAH by carbamylation of the enzyme's serine nucleophile. LY2183240 also inhibits several other brain serine hydrolases with IC50s of 5.3, 0.09, 8.2 nM for MAG lipase, bh6 and KIAA1363, respectively .
|
-

-
- HY-128429S
-
|
(E)-2-Hexenal-d2-1
|
Isotope-Labeled Compounds
Fungal
Biochemical Assay Reagents
|
Infection
|
|
trans-2-Hexenal-d2-1 is the deuterium labeled trans-2-Hexenal (HY-128429). trans-2-Hexenal ((E)-2-Hexenal) is a volatile compound widely present in fresh plants, vegetables, and fruits, with a unique leafy aroma. trans-2-Hexenal has antifungal activity and can also inhibit the germination of soybean seeds and the growth of seedlings. In addition, trans-2-Hexenal can be used to determine low-molecular-weight carbonyl compounds that are reactive with biological nucleophiles in biological samples .
|
-

-
- HY-W002004
-
|
4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl
|
Biochemical Assay Reagents
|
Inflammation/Immunology
|
|
4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-

-
- HY-126637
-
|
|
Bacterial
Fungal
|
Infection
|
|
Marasmic acid is a sesquiterpenoid with unsaturated dialdehyde functionality, first isolated from the Basidiomycete Marasmus conigenus. Marasmic acid has antibacterial, antifungal, cytotoxic and mutagenic activities, and its broad-spectrum activity is related to the α,β-unsaturated aldehyde group. However, its detailed biological mechanism of action has not been clarified. Previous studies have suggested that marasmic acid may exert its effects by reacting with endogenous nucleophiles or forming pyrrole derivatives. This study found that marasmic acid interferes with the membrane sensor histidine kinase MoSln1p of M. oryzae, superactivates the HOG pathway and causes cell death, indicating that its mechanism of action is different from other unsaturated dialdehyde sesquiterpenoids.
|
-

-
- HY-W250129
-
|
|
Biochemical Assay Reagents
|
Others
|
|
2,3,4,5-Tetrafluorobenzoyl chloride is a fluorinated organic compound that belongs to the class of benzoyl chlorides. It is a colorless liquid with a pungent smell and is mainly used as an intermediate in the synthesis of various pharmaceutical and pesticide compounds. 2,3,4,5-Tetrafluorobenzoyl chloride is an acylating agent that can react with a variety of nucleophiles, including amines, alcohols, and thiols, to form amides, esters, or thioesters, respectively. Its unique fluorine-containing structure can impart desired properties to target molecules, such as increased lipophilicity or increased stability against metabolic degradation. However, due to its high reactivity and potential health hazards, proper safety measures and handling procedures must be followed when using this compound.
|
-

-
- HY-W002004S1
-
|
4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17
|
Isotope-Labeled Compounds
Biochemical Assay Reagents
|
Inflammation/Immunology
|
|
4-Amino-TEMPO-d17 (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17) is the deuterium labeled 4-Amino-TEMPO (HY-W002004). 4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-

-
- HY-W002004S
-
|
4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17,15N
|
Isotope-Labeled Compounds
Biochemical Assay Reagents
|
Inflammation/Immunology
|
|
4-Amino-TEMPO-d17, 15N (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17, 15N) is the deuterium labeled 4-Amino-TEMPO-d17 (HY-W002004S1). 4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-

-
-
HY-L915
-
|
|
432 compounds
|
|
Lysine is the second most common target residue used in the design of TCIs and related covalent ligands. Its appeal lies in its abundance in human proteins, which is approximately three times higher than that of cysteine (5.8% vs. 1.9%). This significantly increases the number of proteins suitable for covalent targeting, especially given that many human proteins lack ligandable cysteine residues. Moreover, it has been suggested that functional lysines have a lower probability of being replaced by mutation, as they often play a crucial role in catalysis by acting as bases or nucleophiles. Additionally, lysines are essential for maintaining the structural integrity of proteins and for regulating post-translational modifications (PTMs). Consequently, targeting lysine has garnered significant interest in recent years.
Through careful selection, we constructed a structural filter containing over 110 electrophilic groups. By analyzing the electrophilic fragments selected by the structural filter, we removed any molecules with trivial or undesirable structural features. Ultimately, we obtained 445 fragment molecules which can target lysine residue and can be used for fragment-based covalent drug discovery.
|
| Cat. No. |
Product Name |
Type |
-
- HY-101974
-
|
|
Fluorescent Dyes/Probes
|
|
Biotin-PEG3-Bromide is a short PEG linker featuring a biotin group and a bromide. The bromide is a halogen which is easily displaced by nucleophiles such as alcohols or amines. Alternatively, bromide can be applied in a number of cross-coupling reactions such as in a Suzuki reaction. Biotin is useful for affinity-based applications such as pull-down assays or for ligating with streptavidin proteins.
|
-
- HY-112526
-
|
|
Fluorescent Dyes/Probes
|
|
Thiofluor 623 (Compound 3) is a fluorescent turn-on probe that can be used for the selective sensing and bioimaging of thiols. Thiofluor 623 displays excellent immunity to interference from nitrogen and oxygen nucleophiles. Thiofluor 623 is essentially nonfluorescent in the absence of thiols, which cleave the probe and release the red-emissive donor-acceptor fluorophore (Ex=563 nm, Em=623 nm) .
|
| Cat. No. |
Product Name |
Type |
-
- HY-W002004
-
|
4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl
|
Biochemical Assay Reagents
|
|
4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-
- HY-126944
-
|
2-Amino-N-phenylbenzamide
|
Indicators
|
|
2-Aminobenzamide is a neutral and stable compound used as fluorescent tag, numerously in Glycan analysis. 2-aminobenzamide acts as the starting material for several important reactions like Bargellini reaction as an competent ambident nucleophile. Specifically 2-aminobenzamide and its derivatives are used in the blood coagulation cascade .
|
-
- HY-W250129
-
|
|
Biochemical Assay Reagents
|
|
2,3,4,5-Tetrafluorobenzoyl chloride is a fluorinated organic compound that belongs to the class of benzoyl chlorides. It is a colorless liquid with a pungent smell and is mainly used as an intermediate in the synthesis of various pharmaceutical and pesticide compounds. 2,3,4,5-Tetrafluorobenzoyl chloride is an acylating agent that can react with a variety of nucleophiles, including amines, alcohols, and thiols, to form amides, esters, or thioesters, respectively. Its unique fluorine-containing structure can impart desired properties to target molecules, such as increased lipophilicity or increased stability against metabolic degradation. However, due to its high reactivity and potential health hazards, proper safety measures and handling procedures must be followed when using this compound.
|
| Cat. No. |
Product Name |
Target |
Research Area |
-
- HY-W009177
-
|
|
Glyoxalase (GLO)
|
Others
|
|
S-Methylglutathione is an S-substitued glutathione and a stronger nucleophile than GSH . S-Methylglutathione has inhibitory effect on glyoxalase 1 .
|
| Cat. No. |
Product Name |
Category |
Target |
Chemical Structure |
-
- HY-N0548
-
-

-
- HY-128429
-
-

-
- HY-N0548R
-
|
|
Structural Classification
Natural Products
Source classification
Dicerothamnus rhinocerotis
Umbelliferae
Plants
|
Glutathione S-transferase
|
|
α-Angelica lactone (Standard) is the analytical standard of α-Angelica lactone. This product is intended for research and analytical applications. α-Angelica lactone is a naturally occurring anticarcinogen and an vinylogous nucleophile. α-Angelica lactone can give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives and exhibitselectrophilic trapping at the γ-carbon. α-Angelica lactone exerts strong chemoprotective effects by selective enhancement of glutathione-S-thansferase (GST) and UDP-glucononosyltransferase (UGT) detoxification enzymes .
|
-

-
- HY-128429R
-
-

-
- HY-126637
-
|
|
Structural Classification
Microorganisms
Antibiotics
Source classification
Other Antibiotics
|
Bacterial
Fungal
|
|
Marasmic acid is a sesquiterpenoid with unsaturated dialdehyde functionality, first isolated from the Basidiomycete Marasmus conigenus. Marasmic acid has antibacterial, antifungal, cytotoxic and mutagenic activities, and its broad-spectrum activity is related to the α,β-unsaturated aldehyde group. However, its detailed biological mechanism of action has not been clarified. Previous studies have suggested that marasmic acid may exert its effects by reacting with endogenous nucleophiles or forming pyrrole derivatives. This study found that marasmic acid interferes with the membrane sensor histidine kinase MoSln1p of M. oryzae, superactivates the HOG pathway and causes cell death, indicating that its mechanism of action is different from other unsaturated dialdehyde sesquiterpenoids.
|
-

| Cat. No. |
Product Name |
Chemical Structure |
-
- HY-128429S
-
|
|
|
trans-2-Hexenal-d2-1 is the deuterium labeled trans-2-Hexenal (HY-128429). trans-2-Hexenal ((E)-2-Hexenal) is a volatile compound widely present in fresh plants, vegetables, and fruits, with a unique leafy aroma. trans-2-Hexenal has antifungal activity and can also inhibit the germination of soybean seeds and the growth of seedlings. In addition, trans-2-Hexenal can be used to determine low-molecular-weight carbonyl compounds that are reactive with biological nucleophiles in biological samples .
|
-

-
- HY-W002004S1
-
|
|
|
4-Amino-TEMPO-d17 (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17) is the deuterium labeled 4-Amino-TEMPO (HY-W002004). 4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-

-
- HY-W002004S
-
|
|
|
4-Amino-TEMPO-d17, 15N (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl-d17, 15N) is the deuterium labeled 4-Amino-TEMPO-d17 (HY-W002004S1). 4-Amino-TEMPO (4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl) is a stable nitroxide radical and N-nucleophile based on TEMPO. 4-Amino-TEMPO has superoxide dismutase-mimetic activity, can protect cells from oxidative damage, and has radioprotective effects. 4-Amino-TEMPO is widely used in fields such as biomedicine, materials science, and industrial production. 4-Amino-TEMPO can be used as a spin label to detect free radicals, an oxidation catalyst in industrial production, and an antioxidant stabilizer for polymers, among others .
|
-

| Cat. No. |
Product Name |
|
Classification |
-
- HY-W190836
-
|
|
|
Azide
|
|
Azide-PEG2-Tos is a small PEG linker featuring an azide and a tosylate. Tosylates are good leaving groups which are easily displaced by nucleophiles while azide is a click chemistry handle which is used to react with terminal alkynes or strained cyclooctynes.
|
Your information is safe with us. * Required Fields.
Inquiry Information
- Product Name:
- Cat. No.:
- Quantity:
- MCE Japan Authorized Agent:




















































