Search Result
Results for "
5-Fluorouracil
" in MedChemExpress (MCE) Product Catalog:
15
Isotope-Labeled Compounds
| Cat. No. |
Product Name |
Target |
Research Areas |
Chemical Structure |
-
- HY-90006
-
5-Fluorouracil
Maximum Cited Publications
274 Publications Verification
5-FU
|
Nucleoside Antimetabolite/Analog
HIV
Apoptosis
Endogenous Metabolite
|
Cancer
|
|
5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-90006R
-
|
5-FU (Standard)
|
Reference Standards
Nucleoside Antimetabolite/Analog
HIV
Apoptosis
Endogenous Metabolite
|
Cancer
|
|
5-Fluorouracil (Standard) is the analytical standard of 5-Fluorouracil. This product is intended for research and analytical applications. 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-B0097
-
|
5-Fluorouracil 2'-deoxyriboside
|
Nucleoside Antimetabolite/Analog
DNA/RNA Synthesis
Bacterial
CMV
HSV
Apoptosis
|
Infection
Cancer
|
|
Floxuridine (5-Fluorouracil 2'-deoxyriboside) is a pyrimidine analog and known as an oncology antimetabolite. Floxuridine inhibits Poly(ADP-Ribose) polymerase and induces DNA damage by activating the ATM and ATR checkpoint signaling pathways in vitro. Floxuridine is a extreamly potent inhibitor for S. aureus infection and induces cell apoptosis . Floxuridine has antiviral effects against HSV and CMV .
|
-
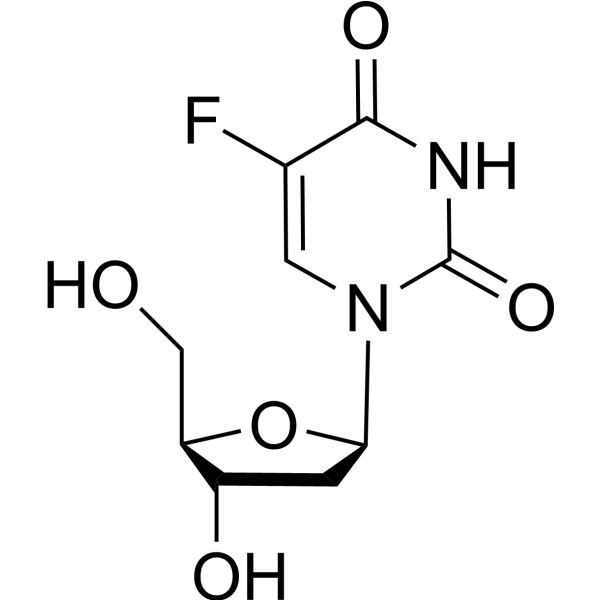
-
- HY-B0097R
-
|
5-Fluorouracil 2'-deoxyriboside (Standard)
|
Reference Standards
Nucleoside Antimetabolite/Analog
DNA/RNA Synthesis
Bacterial
CMV
HSV
Apoptosis
|
Infection
Cancer
|
|
Floxuridine (Standard) is the analytical standard of Floxuridine. This product is intended for research and analytical applications. Floxuridine (5-Fluorouracil 2'-deoxyriboside) is a pyrimidine analog and known as an oncology antimetabolite. Floxuridine inhibits Poly(ADP-Ribose) polymerase and induces DNA damage by activating the ATM and ATR checkpoint signaling pathways in vitro. Floxuridine is a extreamly potent inhibitor for S. aureus infection and induces cell apoptosis . Floxuridine has antiviral effects against HSV and CMV .
|
-

-
- HY-90006S2
-
|
|
Nucleoside Antimetabolite/Analog
HIV
Apoptosis
Endogenous Metabolite
|
Cancer
|
|
5-Fluorouracil- 15N2 is the 15N-labeled 5-Fluorouracil. 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-90006S
-
-

-
- HY-90006S1
-
|
5-FU-13C,15N2
|
Apoptosis
Nucleoside Antimetabolite/Analog
HIV
Endogenous Metabolite
|
Cancer
|
|
5-Fluorouracil- 13C, 15N2 is the 13C and 15N labeled 5-Fluorouracil . 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-W016819B
-
|
5-Fluorouracil-6-carboxylic acid Mono(hydrate); 5-FOA; 5-fluoro OA
|
Thymidylate Synthase
Parasite
|
Infection
|
|
5-Fluoroorotic acid monohydrate is the monohydrate salt form of 5-Fluoroorotic acid (HY-W016819).
5-Fluoroorotic acid monohydrate is the inhibitor for thymidylate synthase that acts as a selective agent in yeast molecular genetics. 5-Fluoroorotic acid monohydrate exhibits antimalarial activity .
|
-

-
- HY-W766548
-
|
5-Fluorouracil 2'-deoxyriboside-13C,15N2
|
Isotope-Labeled Compounds
Apoptosis
Nucleoside Antimetabolite/Analog
CMV
HSV
Bacterial
DNA/RNA Synthesis
|
Cancer
|
|
Floxuridine- 13C, 15N2 (5-Fluorouracil 2'-deoxyriboside- 13C, 15N2) is the 13C- and 15N-labeled labeled Floxuridine (HY-B0097). Floxuridine (5-Fluorouracil 2'-deoxyriboside) is a pyrimidine analog and known as an oncology antimetabolite. Floxuridine inhibits Poly(ADP-Ribose) polymerase and induces DNA damage by activating the ATM and ATR checkpoint signaling pathways in vitro. Floxuridine is a extreamly potent inhibitor for S. aureus infection and induces cell apoptosis . Floxuridine has antiviral effects against HSV and CMV .
|
-

-
- HY-90006S3
-
|
5-FU-13C4,15N2
|
Apoptosis
Nucleoside Antimetabolite/Analog
HIV
Endogenous Metabolite
|
Cancer
|
|
5-Fluorouracil- 13C4, 15N2 is the 13C and 15N labeled 5-Fluorouracil . 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-U00130
-
-

-
- HY-134160
-
|
5-DHFU; 5-Fluorodihydropyrimidine-2,4-dione; 5-Fluorodihydrouracil
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
5,6-Dihydro-5-Fluorouracil (5-DHFU; 5-Fluorodihydropyrimidine-2,4-dione) is the active metabolite of the thymidylate synthase inhibitor prodrug 5-fluorouracil (HY-90006), which is formed from 5-fluorouracil by dihydropyrimidine dehydrogenase (DPD). 5,6-Dihydro-5-Fluorouracil is cytotoxic to HaCaT keratinocytes (IC50=13.5 μM). Intravenous administration of 5,6-Dihydro-5-Fluorouracil (90 mg/kg/wk) in combination with 5-fluorouracil and the DPD inhibitor eniluracil (HY-10533) slows tumor growth in a rat colon cancer model.
|
-

-
- HY-10533
-
|
5-Ethynyluracil; GW776C85
|
Biochemical Assay Reagents
|
Cancer
|
|
Eniluracil (5-Ethynyluracil) is an orally active dihydropyrimidine dehydrogenase (DPD) inhibitor. Eniluracil irreversibly inhibits DPD, increases the oral bioavailability of 5-fluorouracil to 100%, and facilitates the uniform absorption and toxicity of 5-fluorouracil. Eniluracil can be used in cancer research of combination with fluoropyrimidines (including 5-fluorouracil) . Eniluracil is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
|
-

-
- HY-10533R
-
|
5-Ethynyluracil (Standard); GW776C85 (Standard)
|
Biochemical Assay Reagents
Reference Standards
|
Cancer
|
|
Eniluracil (Standard) is the analytical standard of Eniluracil. This product is intended for research and analytical applications. Eniluracil (5-Ethynyluracil) is an orally active dihydropyrimidine dehydrogenase (DPD) inhibitor. Eniluracil irreversibly inhibits DPD, increases the oral bioavailability of 5-fluorouracil to 100%, and facilitates the uniform absorption and toxicity of 5-fluorouracil. Eniluracil can be used in cancer research of combination with fluoropyrimidines (including 5-fluorouracil) . Eniluracil is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
|
-

-
- HY-13667
-
|
Calcium levofolinate; CL307782
|
Antifolate
|
Cancer
|
|
Levoleucovorin Calcium (Calcium levofolinate) is the active form of calcium Folinic acid (HY-13664) and has anti-tumor effects. Levoleucovorin Calcium is also a potentiator of the anti-cancer toxicity of 5-Fluorouracil (HY-90006) .
|
-

-
- HY-13667R
-
|
Calcium levofolinate (Standard); CL307782 (Standard)
|
Reference Standards
Antifolate
|
Cancer
|
|
Levoleucovorin (Calcium) (Standard) is the analytical standard of Levoleucovorin (Calcium). This product is intended for research and analytical applications. Levoleucovorin Calcium (Calcium levofolinate), a levo isoform of Leucovorin Calcium (HY-13664), possesses antineoplastic effects. Levoleucovorin Calcium is also an augmentor of 5-fluorouracil (HY-90006) cytotoxicity against cancer .
|
-

-
- HY-134160S
-
|
5-DHFU-13C,15N2; 5-Fluorodihydropyrimidine-2,4-dione-13C,15N2; 5-Fluorodihydrouracil-13C,15N2
|
Isotope-Labeled Compounds
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
5,6-Dihydro-5-Fluorouracil- 13C, 15N2 (5-DHFU- 13C, 15N2) is the 13C- and 15N-labeled labeled 5,6-Dihydro-5-Fluorouracil (HY-134160). 5,6-Dihydro-5-Fluorouracil (5-DHFU; 5-Fluorodihydropyrimidine-2,4-dione) is the active metabolite of the thymidylate synthase inhibitor prodrug 5-fluorouracil (HY-90006), which is formed from 5-fluorouracil by dihydropyrimidine dehydrogenase (DPD). 5,6-Dihydro-5-Fluorouracil is cytotoxic to HaCaT keratinocytes (IC50=13.5 μM). Intravenous administration of 5,6-Dihydro-5-Fluorouracil (90 mg/kg/wk) in combination with 5-fluorouracil and the DPD inhibitor eniluracil (HY-10533) slows tumor growth in a rat colon cancer model.
|
-

-
- HY-106406R
-
|
BAU (Standard); 5-Benzylacyclouridine (Standard)
|
Drug Derivative
Reference Standards
|
Cancer
|
|
Benzylacyclouridine (Standard) is the analytical standard of Benzylacyclouridine. This product is intended for research and analytical applications. Benzylacyclouridine (BAU) is a potent and specific inhibitor of uridine phosphorylase, the first enzyme in the catabolism of uridine. Benzylacyclouridine can modulate the cytotoxic side effects of 5-fluorouracil (5-FU) and its derivatives .
|
-

-
- HY-106406
-
|
BAU; 5-Benzylacyclouridine
|
Drug Derivative
|
Cancer
|
|
Benzylacyclouridine (BAU) is a potent and specific inhibitor of uridine phosphorylase, the first enzyme in the catabolism of uridine. Benzylacyclouridine inhibits the metabolic activity of UPP1 and the activity of UPP2. Benzylacyclouridine can modulate the cytotoxic side effects of 5-fluorouracil (5-FU) and its derivatives [5].
|
-

-
- HY-W438351
-
|
|
RANKL/RANK
|
Metabolic Disease
|
|
1,3-Dibenzyl-5-fluorouracil is a chemical inhibitor of osteoclastogenesis. 1,3-Dibenzyl-5-fluorouracil inhibits the expression of osteoclast markers by downregulating the receptor activator of NF-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) signaling pathways. 1,3-Dibenzyl-5-fluorouracil can be used in the study of metabolic bone diseases .
|
-

-
- HY-169474
-
|
PAMAM G1.0
|
Biochemical Assay Reagents
|
Cancer
|
|
Starburst 1st Generation (PAMAM G1.0) is a Polyamidoamine (HY-164657; PAMAM) dendrimer with amine termini that has been used as a drug delivery system in vitro. Starburst 1st Generation conjugated to di-n-dodecylamine and encapsulating 5-Fluorouracil (HY-90006) increase the solubility of 5-Fluorouracil and are cytotoxic to AGS gastric adenocarcinoma cells .
|
-

-
- HY-154128
-
|
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
1-(b-D-Xylofuranosyl)-5-fluorouracil is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc .
|
-

-
- HY-134529
-
|
Ribose 1-phosphate
|
Endogenous Metabolite
|
Others
|
|
D-Ribofuranose1-dihydrogenphosphate, also known as ribose 1-phosphate, is the material for the synthesis of 5-fluorouracil (FUra) by uridine phosphorylase .
|
-

-
- HY-134529A
-
|
Ribose 1-phosphate dicyclohexanamine
|
Endogenous Metabolite
|
Others
|
|
D-Ribofuranose1-dihydrogenphosphate dicyclohexanamine, also known as ribose 1-phosphate, is the material for the synthesis of 5-fluorouracil (FUra) by uridine phosphorylase .
|
-

-
- HY-W010450S
-
-

-
- HY-W010450S3
-
|
|
Endogenous Metabolite
DNA/RNA Synthesis
|
Cancer
|
|
Thymine- 13C is the 13C labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W010450S2
-
|
|
Endogenous Metabolite
|
Cancer
|
|
Thymine- 15N2 is the 15N labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W010450R
-
|
|
Reference Standards
Endogenous Metabolite
|
Metabolic Disease
Cancer
|
|
Thymine (Standard) is the analytical standard of Thymine. This product is intended for research and analytical applications. Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM.
|
-

-
- HY-W010450S4
-
|
|
Endogenous Metabolite
|
Cancer
|
|
Thymine-d4-1 is the deuterium labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-109051
-
|
|
Endogenous Metabolite
|
Cancer
|
|
Arfolitixorin is a potent 5-Fluorouracil (5-FU) moderator. Arfolitixorin is an immediately active form of Folate, [6R]-5,10-methylenetetrahydrofolate ([6R]-MTHF). Arfolitixorin is potent for the research of metastatic colorectal cancer .
|
-

-
- HY-109051C
-
|
|
Endogenous Metabolite
|
Cancer
|
|
Arfolitixorin sulfate is a potent 5-Fluorouracil (5-FU) moderator. Arfolitixorin sulfate is an immediately active form of Folate, [6R]-5,10-methylenetetrahydrofolate ([6R]-MTHF). Arfolitixorin sulfate is potent for the research of metastatic colorectal cancer .
|
-

-
- HY-N3316
-
|
|
Others
|
|
|
Martynoside protects ex vivo bone marrow cells from 5-fluorouracil (5-FU)-induced cell death and inflammation response by down-regulating the TNF signaling pathway .Martynoside is a potent antiestrogen in MCF-7 cells, increasing IGFBP3 levels
|
-

-
- HY-W747214
-
|
|
Isotope-Labeled Compounds
Endogenous Metabolite
|
Cancer
|
|
Thymine- 15N2, 13C is the 13C and 15N labeled Thymine (HY-W010450). Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-Fluorouracil (HY-90006) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-145311
-
|
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
Bis-Pro-5FU (Compound 4) is a 5-FU precursor that confers oral bioavailability and increase the safety profile of 5-Fluorouracil (5-FU) chemotherapy regimens. 5-FU is an antineoplastic antimetabolite that is widely used for the research of colorectal and pancreatic cancer .
|
-

-
- HY-112732
-
|
|
Apoptosis
|
Metabolic Disease
Cancer
|
|
Sparfosic acid, a DNA antimetabolite agent, is a potent inhibitor of aspartate transcarbamoyl transferase, the enzyme catalyzing the second step of de novo pyrimidine biosynthesis. Sparfosic acid synergistically enhances the cytotoxicity of a combination of 5-fluorouracil (5-FU) and interferon-alpha (IFN) against human colon cancer cell lines .
|
-

-
- HY-154508
-
|
2’-Deoxy-5-Fluorouridine 5’-phosphate triethylammonium
|
Nucleoside Antimetabolite/Analog
Thymidylate Synthase
|
Cancer
|
|
FdUMP triethylammonium is the intracellular active form of 5-fluorouracil (5-FU). 5-FU is converted to FdUMP after being transported into the cell by various enzymes. FdUMP forms a ternary complex with thymidylate synthase and its cofactor 5,10-methylene tetrahydrofolate, inhibiting the activity of thymidylate synthase, which in turn leads to the suppression of DNA synthesis.
|
-

-
- HY-W010450S5
-
|
5-Methyluacil-13C5,15N,15N2
|
Isotope-Labeled Compounds
Endogenous Metabolite
|
Metabolic Disease
Cancer
|
|
Thymine- 13C5, 15N2 (5-Methyluacil- 13C5, 15N2) is 13C and 15N labeled Thymine. Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-112732B
-
|
|
Apoptosis
|
Metabolic Disease
Cancer
|
|
Sparfosic acid trisodium is a DNA antimetabolite agent and a potent inhibitor of aspartate transcarbamoyl transferase. Aspartate transcarbamoyl transferase catalyzes the second step of de novo pyrimidine biosynthesis. Sparfosic acid trisodium synergistically enhances the cytotoxicity of a combination of 5-fluorouracil (5-FU) and interferon-alpha (IFN) against human colon cancer cell lines .
|
-

-
- HY-14905
-
|
Tri-O-acetyl uridine
|
Drug Derivative
|
Neurological Disease
|
|
Uridine triacetate (Tri-O-acetyl uridine) is an orally active proagent of Uridine (HY-B1449). Uridine triacetate is quickly absorbed in the gut, and is rapidly deacetylated in the circulation to yield free uridine. Uridine triacetate is used for the research of 5-fluorouracil (5-FU) and capecitabine toxicity, or early-onset cardiac or central nervous system (CNS) .
|
-

-
- HY-W010575S
-
|
|
Isotope-Labeled Compounds
|
Others
|
|
(2R)-3-Amino-2-fluoropropanoic acid- 13C3 is a 13C-labeled (2R)-3-Amino-2-fluoropropanoic acid. (2R)-3-Amino-2-fluoropropanoic acid is the catabolism of anticancer agent 5-Fluorouracil .
|
-

-
- HY-129389
-
|
|
Glycosyltransferase
|
Cancer
|
|
Benzyl-α-GalNAc is a potent O-glycosylation inhibitor. Benzyl-α-GalNAc effectively inhibits the proliferation and activation of LX-2 cells and suppresses the expression of collagen I/III, which has good potential for investigation in liver fibrosis. Benzyl-α-GalNAc also significantly enhances the anti-tumour activity of 5-Fluorouracil (HY-90006) (e.g. pancreatic cancer) by inhibiting O-glycosylation .
|
-

-
- HY-163828
-
|
|
Apoptosis
Phosphatase
c-Myc
GSK-3
ERK
Reactive Oxygen Species (ROS)
|
Cancer
|
|
PPA24 is a PP2A activator with a KD of 8.465 μM for PP2ACα. PPA24 induces cancer cell death via apoptosis. PP2ACα induces ROS generation and decreases the level of c-Myc expression. PPA24 can be used to study colorectal cancer (CRC), Folinic acid (HY-17556), 5-Fluorouracil (HY-90006), and Oxaliplatin (HY-17371) (FOLFOX)-resistant CRC, and melanoma cancer .
|
-

-
- HY-W014109
-
|
(E)-5-(2-Bromovinyl)uracil; BVU
|
DNA/RNA Synthesis
|
Infection
|
|
(E)-5-(2-Bromovinyl)uracil (BVU) is a pyrimidine base and an inactive metabolite of the antiviral agents sorivudine and (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVDU) that may be regenerated to BVDU in vivo. BVU irreversibly inactivates dihydropyrimidine dehydrogenase (DPD) in an NADPH-dependent manner. It enhances the efficacy of the chemotherapeutic agent and DPD substrate 5-fluorouracil (HY-90006) in a P388 murine leukemia model when administered at a dose of 200 μmol/kg, increasing survival time.
|
-

-
- HY-172966
-
|
|
EGFR
|
Cancer
|
|
EG31 is an EGFR inhibitor. EG31 effectively inhibits the proliferation of triple-negative breast cancer (TNBC) cells (GI50 values of MDA-MB-231 and Hs578T cells are 498.90 nM and 740.73 nM, respectively) and induces apoptosis by inhibiting the EGFR signaling pathway. EG31 still maintains antiproliferative activity against 5-fluorouracil (5-FU)-resistant TNBC cells (GI50: 519.5 nM). EG31 can be used to study TNBC resistance .
|
-

-
- HY-100958
-
4-DAMP
4 Publications Verification
4-DAMP methiodide
|
mAChR
Apoptosis
MMP
EGFR
Interleukin Related
|
Inflammation/Immunology
Cancer
|
|
4-DAMP (4-DAMP methiodide) is a potent and selective antagonist of M3 receptors and also has a high affinity for the closely-related M5 receptors. 4-DAMP combined with 5-Fluorouracil (5-Fu) (HY-90006) could significantly reduce the cell viability and enhance apoptosis in MKN45 and BGC823 gastric cancer cells. 4-DAMP inhibits lipopolysaccharide (LPS)- and tobacco-induced pulmonary inflammation and reduces mucin 5AC (MUC5AC), oligomeric mucus/gel-forming secretion .
|
-

-
- HY-15815
-
|
|
Epigenetic Reader Domain
Apoptosis
CDK
HIV
|
Cancer
|
|
Bromosporine, a chemical probe, is a potent BET inhibitor with an IC50 value of 2.1 μM for PCAF. Bromosporine can arrest cell cycle and induce apoptosis in cancer cells. Bromosporine exhibits excellent antitumor activity in xenograft mice model when combined with 5-Fluorouracil (HY-90006). Bromosporine can increase CDK9 T-loop phosphorylation in HIV-1 latency models, resulting the protection of reactivate HIV-1 replication from latency. Bromosporine can be used to research colorectal cancer, acute myeloid leukemia (AML) and AIDS .
|
-

-
- HY-103181
-
|
CPA; UK-80882
|
Adenosine Receptor
Apoptosis
|
Cardiovascular Disease
Neurological Disease
Cancer
|
|
N6-Cyclopentyladenosine (CPA) is a selective Adenosine A1 receptor agonist, with Ki values of 2.3 nM, 790 nM and 43 nM for human A1, A2A and A3 receptors, respectively. N6-cyclopentyladenosine increases Apoptosis. N6-Cyclopentyladenosine has antitumor activity against leukemia. N6-cyclopentyladenosine improves 5-fluorouracil (HY-90006)-induced hematopoietic damage, regulates sleep, and delays Aminophylline-induced clonic epileptic seizures [5] .
|
-

-
- HY-16138
-
|
CG-200745
|
HDAC
MDM-2/p53
Apoptosis
|
Cancer
|
|
Ivaltinostat (CG-200745) is an orally active, potent pan-HDAC inhibitor which has the hydroxamic acid moiety to bind zinc at the bottom of catalytic pocket. Ivaltinostat inhibits deacetylation of histone H3 and tubulin. Ivaltinostat induces the accumulation of p53, promotes p53-dependent transactivation, and enhances the expression of MDM2 and p21 (Waf1/Cip1) proteins. Ivaltinostat enhances the sensitivity of Gemcitabine-resistant cells to Gemcitabine (HY-16138) and 5-Fluorouracil (5-FU; HY-90006). Ivaltinostat induces apoptosis and has anti-tumour effects .
|
-

-
- HY-16138A
-
|
CG-200745 formic
|
HDAC
MDM-2/p53
Apoptosis
|
Inflammation/Immunology
Endocrinology
Cancer
|
|
Ivaltinostat (CG-200745) formic is an orally active, potent pan-HDAC inhibitor which has the hydroxamic acid moiety to bind zinc at the bottom of catalytic pocket. Ivaltinostat formic inhibits deacetylation of histone H3 and tubulin. Ivaltinostat formic induces the accumulation of p53, promotes p53-dependent transactivation, and enhances the expression of MDM2 and p21 (Waf1/Cip1) proteins. Ivaltinostat formic enhances the sensitivity of Gemcitabine-resistant cells to Gemcitabine (HY-16138) and 5-Fluorouracil (5-FU; HY-90006). Ivaltinostat formic induces apoptosis and has anti-tumour effects .
|
-

-
- HY-W009538
-
|
5-Fluoro-5'-deoxycytidine
|
Nucleoside Antimetabolite/Analog
DNA/RNA Synthesis
Drug Metabolite
|
Cancer
|
|
5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

- HY-W009538R
-
|
5-Fluoro-5'-deoxycytidine (Standard)
|
Nucleoside Antimetabolite/Analog
Reference Standards
DNA/RNA Synthesis
Drug Metabolite
|
Cancer
|
|
5'-Deoxy-5-fluorocytidine (Standard) is the analytical standard of 5'-Deoxy-5-fluorocytidine (HY-W009538). This product is intended for research and analytical applications. 5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

- HY-W009538S
-
|
5-Fluoro-5'-deoxycytidine-d3
|
Nucleoside Antimetabolite/Analog
Isotope-Labeled Compounds
DNA/RNA Synthesis
Drug Metabolite
|
Cancer
|
|
5'-Deoxy-5-fluorocytidine-d3 is deuterated labeled 5'-Deoxy-5-fluorocytidine (HY-W009538). 5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

- HY-W009538S1
-
|
5-Fluoro-5'-deoxycytidine-13C5
|
Isotope-Labeled Compounds
DNA/RNA Synthesis
Drug Metabolite
|
Cancer
|
|
5'-Deoxy-5-fluorocytidine-13C5 is the 13C labeled isotope of 5'-Deoxy-5-fluorocytidine (HY-W009538). 5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

- HY-107856
-
|
|
Drug Metabolite
DNA/RNA Synthesis
Apoptosis
|
Cancer
|
|
5-Fluorouridine, a metabolite of5-Fluorouracil (HY-90006), is a potent ribozyme self-cleavage inhibitor. 5-Fluorouridine incorporates into both total and poly A RNA and has antiproliferative activity. 5-Fluorouridine induces apoptosis .
|
-

- HY-107856R
-
|
|
Drug Metabolite
Reference Standards
DNA/RNA Synthesis
Apoptosis
|
Cancer
|
|
5-Fluorouridine (Standard) is the analytical standard of 5-Fluorouridine. This product is intended for research and analytical applications. 5-Fluorouridine, a metabolite of5-Fluorouracil (HY-90006), is a potent ribozyme self-cleavage inhibitor. 5-Fluorouridine incorporates into both total and poly A RNA and has antiproliferative activity. 5-Fluorouridine induces apoptosis .
|
-

- HY-19514
-
|
|
Nucleoside Antimetabolite/Analog
|
Cancer
|
|
Fosfluridine Tidoxil is comprised of a specific carrier molecule, coupled through a phosphate group to 5-Fluorouridine (5-FUrd), a metabolite of 5- Fluorouracil (5-FU). Fosfluridine Tidoxil is a oral active anticancer agent .
|
-

- HY-157543
-
|
|
Microtubule/Tubulin
Apoptosis
|
Cancer
|
|
Tubulin polymerization-IN-59 is a tubulin polymerization inhibitor and colchicine binding site inhibitor (CBSI) (IC50 = 6.1 μM). Tubulin polymerization-IN-59 exerts potent antiproliferative activity against cancer cells, while showing lower cytotoxicity to normal cells. Tubulin polymerization-IN-59 arrests colorectal cancer HCT 116 cells in G2/M phase, induces cell apoptosis, and suppresses tumor cell colony formation and migration. Tubulin polymerization-IN-59 can be used for the study of colorectal cancer (CRC) .
|
-

- HY-P2496
-
|
|
Endothelin Receptor
|
Cardiovascular Disease
|
|
Endothelin 1 (swine, human), Alexa Fluor 488-labeled is a synthetic Endothelin 1 peptide labled with Alexa Fluor 488. Endothelin 1 (swine, human) is a synthetic peptide with the sequence of human and swine Endothelin 1, which is a potent endogenous vasoconstrictor. Endothelin 1 acts through two types of receptors ETA and ETB .
|
-

- HY-13867AR
-
|
|
PKC
GSK-3
|
Metabolic Disease
Cancer
|
|
Bisindolylmaleimide I (hydrochloride) (Standard) is the analytical standard of Bisindolylmaleimide I (hydrochloride). This product is intended for research and analytical applications. Bisindolylmaleimide I (GF109203X) hydrochloride is a cell-permeable and reversible PKC inhibitor (IC50 of 20 nM, 17 nM, 16 nM, and 20 nM for PKCα, PKCβI, PKCβII, and PKCγ. Bisindolylmaleimide I hydrochloride is also a GSK-3 inhibitor .
|
-

- HY-13867A
-
|
GF109203X hydrochloride; Go 6850 hydrochloride
|
PKC
GSK-3
|
Metabolic Disease
Cancer
|
|
Bisindolylmaleimide I (GF109203X) hydrochloride is a cell-permeable and reversible PKC inhibitor (IC50 of 20 nM, 17 nM, 16 nM, and 20 nM for PKCα, PKCβI, PKCβII, and PKCγ. Bisindolylmaleimide I hydrochloride is also a GSK-3 inhibitor .
|
-

- HY-13867R
-
|
|
PKC
GSK-3
|
Metabolic Disease
Cancer
|
|
Bisindolylmaleimide I (Standard) is the analytical standard of Bisindolylmaleimide I. This product is intended for research and analytical applications. Bisindolylmaleimide I (GF109203X) is a cell-permeable and reversible PKC inhibitor (IC50 of 20 nM, 17 nM, 16 nM, and 20 nM for PKCα, PKCβI, PKCβII, and PKCγ. Bisindolylmaleimide I is also a GSK-3 inhibitor .
|
-

- HY-13867
-
|
GF109203X; Go 6850
|
PKC
GSK-3
|
Metabolic Disease
Cancer
|
|
Bisindolylmaleimide I (GF109203X) is a cell-permeable and reversible PKC inhibitor (IC50 of 20 nM, 17 nM, 16 nM, and 20 nM for PKCα, PKCβI, PKCβII, and PKCγ. Bisindolylmaleimide I is also a GSK-3 inhibitor .
|
-

- HY-P5372
-
|
|
Protease Activated Receptor (PAR)
|
Cancer
|
|
Ala-parafluoroPhe-Arg-Cha-Cit-Tyr-NH2, a bioactive peptide, is a selective Protease activating receptor 1 (PAR-1) agonist over PAR-2. PAR-1 belongs to a subfamily of G-protein coupled receptors and is known to mediate the cellular effects of thrombin. In addition to its varied cellular effects of thrombin, PAR-1 has also been shown to coordinate with PAR-4 and regulate thrombin-induced hepatocellular carcinoma harboring thrombin formation within the tumor environment classified as 'coagulation type' .
|
-

- HY-P5372A
-
|
|
Protease Activated Receptor (PAR)
|
Cancer
|
|
Ala-parafluoroPhe-Arg-Cha-Cit-Tyr-NH2 TFA, a bioactive peptide, is a selective Protease activating receptor 1 (PAR-1) agonist over PAR-2. PAR-1 belongs to a subfamily of G-protein coupled receptors and is known to mediate the cellular effects of thrombin. In addition to its varied cellular effects of thrombin, PAR-1 has also been shown to coordinate with PAR-4 and regulate thrombin-induced hepatocellular carcinoma harboring thrombin formation within the tumor environment classified as 'coagulation type' .
|
-

- HY-109115
-
|
NUC-3373
|
Thymidylate Synthase
|
Cancer
|
|
Fosifloxuridine nafalbenamide (NUC-3373), a pyrimidine nucleotide analogue, is a Thymidylate synthase inhibitor. Fosifloxuridine nafalbenamide has anticancer activity. Fosifloxuridine nafalbenamide has the potential to evoke a host immune response and enhance immunoresearch .
|
-

| Cat. No. |
Product Name |
Target |
Research Area |
-
- HY-P5372A
-
|
|
Protease Activated Receptor (PAR)
|
Cancer
|
|
Ala-parafluoroPhe-Arg-Cha-Cit-Tyr-NH2 TFA, a bioactive peptide, is a selective Protease activating receptor 1 (PAR-1) agonist over PAR-2. PAR-1 belongs to a subfamily of G-protein coupled receptors and is known to mediate the cellular effects of thrombin. In addition to its varied cellular effects of thrombin, PAR-1 has also been shown to coordinate with PAR-4 and regulate thrombin-induced hepatocellular carcinoma harboring thrombin formation within the tumor environment classified as 'coagulation type' .
|
-
- HY-P5373
-
|
|
Peptides
|
Others
|
|
Ser-parafluoroPhe-Aad-Leu-Arg-Asn-Pro-NH2 is a biological active peptide. (Structure-activity studies of thrombin receptor-tethered ligand SFLLRNP have revealed
the importance of the Phe-2-phenyl group in receptor recognition and the replacement of the
Phe-2 by para-fluorophenylalanine [(p-F)Phe] was found to enhance its activity)
|
-
- HY-P2496
-
|
|
Endothelin Receptor
|
Cardiovascular Disease
|
|
Endothelin 1 (swine, human), Alexa Fluor 488-labeled is a synthetic Endothelin 1 peptide labled with Alexa Fluor 488. Endothelin 1 (swine, human) is a synthetic peptide with the sequence of human and swine Endothelin 1, which is a potent endogenous vasoconstrictor. Endothelin 1 acts through two types of receptors ETA and ETB .
|
-
- HY-P5372
-
|
|
Protease Activated Receptor (PAR)
|
Cancer
|
|
Ala-parafluoroPhe-Arg-Cha-Cit-Tyr-NH2, a bioactive peptide, is a selective Protease activating receptor 1 (PAR-1) agonist over PAR-2. PAR-1 belongs to a subfamily of G-protein coupled receptors and is known to mediate the cellular effects of thrombin. In addition to its varied cellular effects of thrombin, PAR-1 has also been shown to coordinate with PAR-4 and regulate thrombin-induced hepatocellular carcinoma harboring thrombin formation within the tumor environment classified as 'coagulation type' .
|
| Cat. No. |
Product Name |
Category |
Target |
Chemical Structure |
| Cat. No. |
Product Name |
Chemical Structure |
-
- HY-90006S2
-
|
|
|
5-Fluorouracil- 15N2 is the 15N-labeled 5-Fluorouracil. 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-90006S
-
|
|
|
5-Fluorouracil-d is the deuterium labeled 5-Fluorouracil. 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-90006S1
-
|
|
|
5-Fluorouracil- 13C, 15N2 is the 13C and 15N labeled 5-Fluorouracil . 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-W010450S4
-
|
|
|
Thymine-d4-1 is the deuterium labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W766548
-
|
|
|
Floxuridine- 13C, 15N2 (5-Fluorouracil 2'-deoxyriboside- 13C, 15N2) is the 13C- and 15N-labeled labeled Floxuridine (HY-B0097). Floxuridine (5-Fluorouracil 2'-deoxyriboside) is a pyrimidine analog and known as an oncology antimetabolite. Floxuridine inhibits Poly(ADP-Ribose) polymerase and induces DNA damage by activating the ATM and ATR checkpoint signaling pathways in vitro. Floxuridine is a extreamly potent inhibitor for S. aureus infection and induces cell apoptosis . Floxuridine has antiviral effects against HSV and CMV .
|
-

-
- HY-90006S3
-
|
|
|
5-Fluorouracil- 13C4, 15N2 is the 13C and 15N labeled 5-Fluorouracil . 5-Fluorouracil (5-FU) is an analogue of uracil and a potent antitumor agent. 5-Fluorouracil affects pyrimidine synthesis by inhibiting thymidylate synthetase thus depleting intracellular dTTP pools. 5-Fluorouracil induces apoptosis and can be used as a chemical sensitizer . 5-Fluorouracil also inhibits HIV .
|
-

-
- HY-134160S
-
|
|
|
5,6-Dihydro-5-Fluorouracil- 13C, 15N2 (5-DHFU- 13C, 15N2) is the 13C- and 15N-labeled labeled 5,6-Dihydro-5-Fluorouracil (HY-134160). 5,6-Dihydro-5-Fluorouracil (5-DHFU; 5-Fluorodihydropyrimidine-2,4-dione) is the active metabolite of the thymidylate synthase inhibitor prodrug 5-fluorouracil (HY-90006), which is formed from 5-fluorouracil by dihydropyrimidine dehydrogenase (DPD). 5,6-Dihydro-5-Fluorouracil is cytotoxic to HaCaT keratinocytes (IC50=13.5 μM). Intravenous administration of 5,6-Dihydro-5-Fluorouracil (90 mg/kg/wk) in combination with 5-fluorouracil and the DPD inhibitor eniluracil (HY-10533) slows tumor growth in a rat colon cancer model.
|
-

-
- HY-W010450S
-
|
|
|
Thymine-d4 is the deuterium labeled Thymine. Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM.
|
-

-
- HY-W010450S3
-
|
|
|
Thymine- 13C is the 13C labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W010450S2
-
|
|
|
Thymine- 15N2 is the 15N labeled Thymine . Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W747214
-
|
|
|
Thymine- 15N2, 13C is the 13C and 15N labeled Thymine (HY-W010450). Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-Fluorouracil (HY-90006) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W010450S5
-
|
|
|
Thymine- 13C5, 15N2 (5-Methyluacil- 13C5, 15N2) is 13C and 15N labeled Thymine. Thymine is one of the four nucleobases in the nucleic acid of DNA and can be a target for actions of 5-fluorouracil (5-FU) in cancer treatment, with a Km of 2.3 μM .
|
-

-
- HY-W010575S
-
|
|
|
(2R)-3-Amino-2-fluoropropanoic acid- 13C3 is a 13C-labeled (2R)-3-Amino-2-fluoropropanoic acid. (2R)-3-Amino-2-fluoropropanoic acid is the catabolism of anticancer agent 5-Fluorouracil .
|
-

-
- HY-W009538S
-
|
|
|
5'-Deoxy-5-fluorocytidine-d3 is deuterated labeled 5'-Deoxy-5-fluorocytidine (HY-W009538). 5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

-
- HY-W009538S1
-
|
|
|
5'-Deoxy-5-fluorocytidine-13C5 is the 13C labeled isotope of 5'-Deoxy-5-fluorocytidine (HY-W009538). 5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-

| Cat. No. |
Product Name |
|
Classification |
-
- HY-10533
-
|
5-Ethynyluracil; GW776C85
|
|
Alkynes
|
|
Eniluracil (5-Ethynyluracil) is an orally active dihydropyrimidine dehydrogenase (DPD) inhibitor. Eniluracil irreversibly inhibits DPD, increases the oral bioavailability of 5-fluorouracil to 100%, and facilitates the uniform absorption and toxicity of 5-fluorouracil. Eniluracil can be used in cancer research of combination with fluoropyrimidines (including 5-fluorouracil) . Eniluracil is a click chemistry reagent, it contains an Alkyne group and can undergo copper-catalyzed azide-alkyne cycloaddition (CuAAc) with molecules containing Azide groups.
|
| Cat. No. |
Product Name |
|
Classification |
-
- HY-154508
-
|
2’-Deoxy-5-Fluorouridine 5’-phosphate triethylammonium
|
|
Nucleoside Analogs
Uridine
|
|
FdUMP triethylammonium is the intracellular active form of 5-fluorouracil (5-FU). 5-FU is converted to FdUMP after being transported into the cell by various enzymes. FdUMP forms a ternary complex with thymidylate synthase and its cofactor 5,10-methylene tetrahydrofolate, inhibiting the activity of thymidylate synthase, which in turn leads to the suppression of DNA synthesis.
|
-
- HY-W009538
-
|
5-Fluoro-5'-deoxycytidine
|
|
Nucleoside Analogs
Cytidine
|
|
5'-Deoxy-5-fluorocytidine (5-Fluoro-5'-deoxycytidine) is a cytidine analog and metabolite of Capecitabine (HY-B0016). 5'-Deoxy-5-fluorocytidine is converted from Capecitabine by carboxylesterase in the liver. 5'-Deoxy-5-fluorocytidine is deaminated by cytidine deaminase to generate 5'-deoxy-5-fluorouridine, which is finally converted into 5-fluorouracil (HY-90006) by thymidine phosphorylase in tumor tissues to exert anti-tumor effects. 5'-Deoxy-5-fluorocytidine is used in the researches for solid tumors such as colorectal cancer, non-small cell lung cancer and breast cancer .
|
-
- HY-154128
-
|
|
|
Nucleoside Analogs
Uridine
|
|
1-(b-D-Xylofuranosyl)-5-fluorouracil is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc .
|
-
- HY-19514
-
|
|
|
Nucleoside Analogs
Uridine
|
|
Fosfluridine Tidoxil is comprised of a specific carrier molecule, coupled through a phosphate group to 5-Fluorouridine (5-FUrd), a metabolite of 5- Fluorouracil (5-FU). Fosfluridine Tidoxil is a oral active anticancer agent .
|
Your information is safe with us. * Required Fields.
Inquiry Information
- Product Name:
- Cat. No.:
- Quantity:
- MCE Japan Authorized Agent: